 You can read my previous posts on this drug here (1, 2).
You can read my previous posts on this drug here (1, 2).The Research: Part 2
The second study published on the efficacy of agomelatine was by Kennedy and Emsley (2006, 3).
This was a 6-week, double-blind, randomized, placebo-controlled study involving 212 patients. Dosage ranged from 25-50mg/day (dose adjustment at week 2 for poor responders). No other active comparator (e.g., paroxetine) was used in this study. Similar to the previous study (Loo et al, 2002), the efficacy of agomelatine on a severely depressed subpopulation was examine too.
Surprise, surprise, agomelatine was shown to be superior to placebo (HAM-D total score 14.1 +/- 7.7 versus 16.5+/- 7.4). Plot twist: "The proportion of patients who were in remission by the end of the acute treatment period was not statistically different between the two treatment groups." Of course, that could be due to the short duration (6-weeks) of the study.
Remember this quote from the previous study I reviewed: "25mg of agomelatine was significantly better than placebo at 2 weeks..., whereas this significant advantage for paroxetine...did not emerge until 4 weeks." Here is the survival analysis for this study:
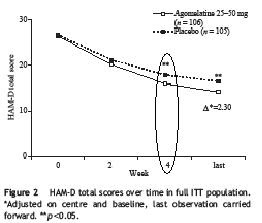 The difference did not occur until week 4, the same as paroxetine in the previous study. So this study failed to replicate the result of the first study.
The difference did not occur until week 4, the same as paroxetine in the previous study. So this study failed to replicate the result of the first study.Common side-effects reported include: "dizziness, nasopharyngitis and influenza were more common in the agomelatine group that placebo." Again, no sexual side-effects were reported (sorry, no fancy chart to show).
Part 3:
The third published study was by Olie and Kasper (2005, 4). This study is similar in design as the study mentioned-above. At the end of 6-weeks, there was a superior response for agomelatine compared to placebo (3.44 point difference).
Here is the survival analysis curve for time to first response:
 Here, you can see a difference was noted at week 2 (replicating the original result), but then they merge at week 4 (difference was still significant) and then separate again thereafter. What is interesting about placebo temporarily merging with the active drug at week four, is that there was a dose adjustment from 25mg to 50mg for poor responders at week 2. Probably not the robust result they were looking for, but a reaction non-the-less.
Here, you can see a difference was noted at week 2 (replicating the original result), but then they merge at week 4 (difference was still significant) and then separate again thereafter. What is interesting about placebo temporarily merging with the active drug at week four, is that there was a dose adjustment from 25mg to 50mg for poor responders at week 2. Probably not the robust result they were looking for, but a reaction non-the-less. Reported side-effects are similar to the previous studies:
Reported side-effects are similar to the previous studies: Comment: Both of these study are extremely short (6-weeks). 2/3 of depressed patients usually do not respond to their first anti-depressant. Moreover, while response rates (50% reduction in symptoms) are usually robust, remissions rates a paltry (usually 1/5-1/3 remission). No long-term information can be gathered from these two short-term studies. There is long-term data, but it's unpublished.
Comment: Both of these study are extremely short (6-weeks). 2/3 of depressed patients usually do not respond to their first anti-depressant. Moreover, while response rates (50% reduction in symptoms) are usually robust, remissions rates a paltry (usually 1/5-1/3 remission). No long-term information can be gathered from these two short-term studies. There is long-term data, but it's unpublished.Side-effect do appear mild. However, many SSRI antidepressant trials show mild side-effects. It's not until the drug is widely prescribe do common side-effects become evident.
All three studies were biased against placebo (i.e., 1 week placebo wash-out period).
Keep in mind that these are published studies of positive trials. There are negative trials that are simply not published (I'm shocked!).
The European Medicines Agency, the parallel to the FDA, initially rejected the drug in 2006 (5).
Here is what they said:
 In case you cannot read the image, it says, "The major concern of the CHMP was that the effectiveness of Valdoxan/Thymanax had not been sufficiently shown. The long-term study (the unpublished data I mentioned) did not show that the medicine was effective. The short-term studies shown that the medicine has an effect, but the extent of this did not allow the Committee to draw a firm conclusion on the medicine's effectiveness."
In case you cannot read the image, it says, "The major concern of the CHMP was that the effectiveness of Valdoxan/Thymanax had not been sufficiently shown. The long-term study (the unpublished data I mentioned) did not show that the medicine was effective. The short-term studies shown that the medicine has an effect, but the extent of this did not allow the Committee to draw a firm conclusion on the medicine's effectiveness."The drug was finally approved in 2008 (6). In their report they list all the submitted trials.

 Some highlights:
Some highlights:-In study CL3-22, which included a fluoxetine comparator. This study, which was a short-term with a long-term (1 year) extension found that both agomelatine and fluoxetine were not statistically superior to placebo. (oops!).
-In study CL3-23 agomelatine and paroxetine were not statistically superior to placebo over the short-and-long term. (whoops!).
-CL3-24, the results were identical to CL3-33. (strike three, you're out!).
-Study CL3-21 was a relapse prevention study against placebo. At the end of the trial, agomelatine had a relapse rate of 26% versus 24% for the placebo group (strike four! wait that's not right). They did a post-hoc analysis (i.e., statistical masturbation) and found that only for severely depressed patients there was a statistical difference. The proper thing to do at this point is to run a NEW study to test that intriguing hypothesis since the analysis was done after the fact. (It didn't happen, obviously).
-Efficacy in the elderly was not demonstrated
-Because of concerns over liver toxicity, liver monitoring is required. (do they require that for SSRI's)
Versus other Antidepressants
Much of the hoopla around this drug has been it's supposed superiority against fluoxetine (Prozac). If you head over to the official website, they tout the findings of a recent study (7). But is it really superior? The data submitted to the EMEA showed agomelatine to be equal to SSRI's (2 paroxetine studies, 2 fluoxetine studies, & 2 venlafaxine studies). With the exception of one study where superiority to sertraline (submitted later) was shown. Here's is what the EMEA had to say about the matter:
 "magnitude appears less than the active comparators."So that's 2 studies out of 8 that showed a superior effect. There are many studies in the literature that show one antidepressant being superior to another (8). However, results like theses are the exception, not the rule.
"magnitude appears less than the active comparators."So that's 2 studies out of 8 that showed a superior effect. There are many studies in the literature that show one antidepressant being superior to another (8). However, results like theses are the exception, not the rule.The Hype
Based on my review of the data, I'm not seeing much in the way of a wonderful new addition to the anti-depressant family. Aside from liver toxicity, side-effect profile does seem favorable, which is certainly an advantage compared to SSRI's. However, efficacy does not appear any greater than currently available treatments (maybe less effective overall). Just like SSRI's, there are a number of negative trials, so the effect is certainly not consistent.
Furthermore, during my review, I found 6 review articles (see my first post), which rehash the same 3 primary studies over and over again. What's worse, these 6 articles were published within a 3 year period and all in the journals for which Montgomery is the editor. They also read like the democratic party's "talking points" on health care reform, meaning, they all stay on message. That message being "need for better antidepressants" "safety and tolerability" "unique mechanism of action." This strikes me as familiar to the recent trend in second generation antipsychotic articles (9, 10, 11). What I truly enjoyed, though, is the SSRI bashing that was going on in these studies. Last Psychiatrist discussed quite well last year (12, 13).
My Final Verdict
Slightly better side-effect profile, actual clinical efficacy is uncertain.
KENNEDY, S., & EMSLEY, R. (2006). Placebo-controlled trial of agomelatine in the treatment of major depressive disorder European Neuropsychopharmacology, 16 (2), 93-100 DOI: 10.1016/j.euroneuro.2005.09.002
Pierre Olié, J., & Kasper, S. (2007). Efficacy of agomelatine, a MT1/MT2 receptor agonist with 5-HT2C antagonistic properties, in major depressive disorder The International Journal of Neuropsychopharmacology, 10 (05) DOI: 10.1017/S1461145707007766





11 comments:
I think the drug inustry will soon learn nobody believes their testing any more they have lied and injured/killed too many for too long. Most people I talk to will no longer take a drug unless there are life or death even then it is debatable WE NO LONGER TRUST THEM OR THEIR DRUGS
I just found this blog and I really liked the article because of the depth of actual review. I am in the business of drug development and yes frustrations abound...but there are many with the proper moral compass (in a day where drug development can be halted by a report of a SAE (Serious Adverse Event)by an unqualified entity and is miss-interpreted, leading to FDA acting in a way reminiscent of being put on Jessie Jackson and sweatsuit sideshow's hit-list), I take any "interpretation blog" with a grain of salt and so far am impressed with the angle of this blog's article. I am commenting just because the comment before mine. As a friend of mine said recently to her patient who is on dialysis (and in non-compliant) who was saying the new "Obamacare" was going to be so great..."if it (Obamacare) goes through we will not be seeing you here anymore Mr. XXXXXXX because your insurance has allowed you to be non-compliant and remain in treatment but you will have already been outside the guidelines for treatment". I have to laugh when the poster says he would only use for matters of life or death, well if it is a matter of life or death...treatment WILL NOT EVEN BE AVAILABLE. The major pharmas have already cut huge amounts of drugs in pre-clinical and Phase 1 or Phase 2 development if they are not for "general conditions" and that is in anticipation of this healthcare mess. Just got me angry because typical zombie under the spell. I will read more of the blog when I have a chance (great article).
If you look at the very simple structure of this drug the primary ring structure is a naphthalene. Even the IUPAC name, N-[2-(7-methoxynaphthalen-1-yl)ethyl]acetamide, indicates this.
Naphthalenes are extremely toxic especially upon metabolism or when exposed to acid as they form napthol (that's right naphthol better known as mothballs).
Another drug based on a naphthalene is Lilly's Cymbalta it's even enteric coated to prevent formation of naphthol in the stomach.
Cymbalta is an incredibly hepatotoxic drug.
Single SAEs in drug development typically do not stop drug development or approvals.
You have to consider the severity of the toxicity itself, such as liver failure which may necessitate transplant or will kill you.) You also have to consider structural signals, and who's at greater risk (alcholics and drug abusers) and if those patients were systematically excluded and if they are actually a large segment of population that will use the drug (drug addicts and alcoholics with depression).
You also have to consider duration of treatment for toxicities that may be cumulative such as 6 week trials with a drug that causes hepatotoxicity with cumulative use. You then also need to look at signals of toxicity that might be harbingers of long term toxicities, such as elevated liver enzymes indicating cell death whose incidence is proportional to the dosages used in the studies.
You also have to consider the likely numbers of people who will have these severe toxicities and if they are offset by the severity of the illness and if there are other safer drugs or not.
There are also many other factors that need to be considered.
Of course whenever anyone tries to raise potential concerns companies will often attack. Companies always over promote efficacy and downplay toxicities and design studies that way.
Drugs are inherently dangerous, sometimes you can predict big problems (like with Vioxx) that won't be apparent until much later after marketing. The problem is suppressing knowledge of the safety signals, not informing patients or prescribers, and then when the deaths start piling up covering them up and doing everything you can to attack and discredit the source.
Salmon
I've been aware of this drug since last year when it was mentioned on one of the Mawdesley Debates, and have been following its progress since.
On placement on a psychiatric ward (I'm in my final year as a student mental health nurse), I've sporadically been asking consultants and pharmacist what their views of it were. Up until today, none of these individuals had heard of it. This afternoon, one of the consultants said they'd been advertising it quite hard. He was very cynical about it. His reasons were that liver tests needed to be conducted at 4, 8 and 12 weeks. He said he doesn't mind this for (his words) "gold standard treatments" like clozapine and lithium, but was not prepared to do it for this drug. I guess that fair enough, particularly as he believes there are adequate treatments "and augmentations" out there already. One thing he did mention that I thought might not be accurate was that "it only works in the dark and patients have the lights on or be out in the daylight". Because agomelatine is a partial melatonin agonist, its effects of improving sleep would not require darkness? Isn't it the case that the brain secretes melatonin when it's dark but the drug is introducing a melatonin like substance to the brain which mimics the action of melatonin, therefore no darkness required?
I'd be interested to hear what you make of his comments.
Paul
Salmon,
Thanks again for your input. You're a great investigator.
Paul,
The melatonergic effects of Valdoxan work just like Rozerem (ramelteon), another melatonergic agonist. They stimulate the M1/M2 receptors in the SCN of the hypothalamus, which cause a high turnover of melatonin, so the reduction of light is not required when the SCN is being directly stimulated.
Perhaps he is confused because the drug needs to be taken in the evening before bed, not because that will allow melatonin to be secreted, but due to the hypnotic effects of the drug itself.
I have to admit that as a U.S. patient I was sort of taken in by the hype and am happy to have your information to damper down my unjustified enthusiasm.
I take prozac now for bipolar II and don't find that it does all that much, though there are probably subtle effects.
Lamotrogine has been a godsend for me and really has helped my cognition in a way that sertraline which was very effective in getting me out of a moderate/major depression. For anxiety I do take klonopin, but by far the most useful thing has been a low dose of propranalol. Propranalol and Lamictal make psychotherapy possible.
I wish that I could go off the prozac, because I'd like the sexual side effects to go away.
And the big thing is that I still have sleep disturbances which can affect my circadian rhythms in a big way. I have a CPAP machine to treat sleep hypopnea (and I'm not voerweight--just petite with a small neck and a large chest), but if I could find something that really helped with sleep quality and had some antidepressant effects, I'd be really happy. Because my biggest residual symptom is one which is unlikely to show up on a HAM-D score: bouts of debilitating exhaustion. It does, however, severely affect my anbility to function in a work environment, and I would love to find something to help with it.
My sleep octor is very much a behaviorist and would be loath to prescribe any drugs, plus I don't get the sense that teh currently available ones do anything to improve sleep architecture.
That's a long way of saying that I desperately want agomelatine to be a miracle drug, and your dose of reality is helpful.
Most drugs alter sleep architecture instead of restoring; however, the hypnotics such as zolpidem etc don't alter sleep architecture (though they are only good for sleep initiation). Stronger benzos like clonazepam do alter sleep architecture as do SSRI antidepressants (supress REM sleep).
If you believe that Prozac isnt doing anything, it sounds as if you should discuss with your psychiatrist about switching to a nonSSRI anti-depressant (e.g., buproprion) or coming off it all together and see how you manage with Lamictal and propranalol. Just remember coming off a SSRI has its own problems (sometimes severe problems) and could lead to a worsening of your condition and it can be a lengthy process (up to a year), which is why you should do it with doctor supervision. Just remember, it's your health, so you should advocate for it.
Ive tried Fluoxetine a lot of times and everytime it leads to remission from the most acute symptoms. Once I even bought it online and start eating without supervision because I thought I've had enough of those reoccuring symptoms which some would bitch about was Withdrawal Symptoms and not... oh fuck it let me put it plainly if they only work for work gravely depressed people then I am/was one. There are still shit that needs to be done. The Augmention with wellbutrin XR seemed to be a godsend until i developed hives and had to stop it..
What is particular with Agomelatine is the 5ht2c-antagonism -which it shares with fluoxetine, which makes it's an so called NDDI
(Stephen Stahl coined the term, though it seems more like Serotoninantagonism describes it better because I don't dont see where the disinhibitation of Dopamine and Noradrenaline would come from. So it seems more like its less Serotonin and more N/D from striatium(?))
I guess bupropion/Wellbutrin doesnt have any antianxiolytic potential at all so if Anxiety then Fluoxetin or rather Celexa (or paroxetine if one can stand the antihistaminergic effect of them) would be better.
But of course if Melatonin1 and 2 agonism don't fuck up anything unforeseen which isnt too uncommon with psychiatric drugs would it be bad not suffering from depression? probably no prolactation rise as that goes hand in hand with Serotonin release. no numb dick or erectile dysfunctions, no SSRI apathy and no weight gain ? I think it sounds like a fricking good treat imho.
That would be interesting to test: Beta blocker for calming the amygdala (avoidant PD here) combined with the 5ht2c-antagonism and see how well I can manage without the Serotonin.
Thanks for pointing out the issue with REMsleep as well when it comes SSRIs NeuroPsych15.
Hm it's good Servier Hellas gets a foot in the market. Both Tianeptine and Amineptine was withdrawn from the market due to too dubios reasons.. Amineptine = low risk of abuse, Tiapetine = some stupid fucks tried to inject it and lost their limbs. The withdrawal of Serzone was quite shitty to btw ONE of 250 000 could get liver damage from it. Of course if it's irreversible and acute it's not funny but what about all the shit with Neuroleptics. Diabetes, Tardive Dyskinesia, Altered metabolism (of course to the worse).
Best Regard Selfrenowned PHD Martin
I'm a patient who's been told my whole life that I need to be on some sort of antidepressant, even though I personally don't believe I'm really depressed. Since age 12 when I was put on citalopram "cipramil" as they call it here. Problem is I'm very sensitive to SSRI and SNRI side effects and after a while I refuse to take them because of the physical pain. I do have very obvious mental problems, and have had most my life, but I just don't think they can be called depression.
Now the last thing that the doctor has given me, is this "valdoxan" agometaline, I have not yet had enough time to say if it has had any noticeable effect on me at all, but at least it doesn't give me any noticeable side effect, and it increases my compliance with my doctor (who always wants me on an antidepressant) which is a good thing in psychiatry. Sadly the medicine is not subsidized like all other medicine in Sweden, because it's not really approved, so I have to pay very much (20% of my monthly income) for something that gives me nothing.
But mostly, I worry about the liver toxicity, I have within this last year had some liver issues and had my gall bladder removed in an emergency procedure. I have also developed bad alcohol habit the last year, self medicating with booze against anxiety, because they can't trust me to be given benzoes since I'm a 29 year old male with history of drug abuse. So I drink, or in the rare case that I'm able to get hold of it, take benzoes or zolpidem illegally (and I am very open about this with my doctor). My doctor does not say testing liver values is necessary... so I don't really know if this is a problem, but I am very very afraid of liver damage. Should I really continue taking this medication even if my doctor refuses to take liver tests?
Thank you for the interesting study data, I can't find such things on my own, I wish such articles could be freely available to the public, when I was in a University I had an account that I could access all sorts of such data freely, whether it was relevant to my field of study or not. I can't see why they can't just let everyone have it. I know this post is almost 3 years old, take it as a "bump".
Hi Gunnar. I am also from Sweden. "Funnily" enough Ive also had an gallbladder operation. I was quite yellow and had puked for 3 days before I was approved. I Also had a period of extreme anxiety which I selfmedicated with alcohol. I told my doc it would probably be a better idea if he prescribed benzodiazepines and I had an diagnosis of "anxiety disorder" so he did. Clonazepam. There's different reason why people drink but sadly the industry doesnt seem smart enough to realize that. They always assume alcoholism is about getting a mild opiate therefore the only approved treatment is Naloxone which blocks the opioid-receptors.
Karolinska at this year came out with the "sensational" news that a new drug which increases dopamine could help against alcoholism. (no shit). Some people drink because of low self esteem (dopamine , Bupropion might help), some drinks to relieve themselves from (anxiety) GABA (benzodiazepine) another group drinks for getting the opiatic effect (Naltrexone)
Alcohol is also acting antagonistic on the glutamatereceptors, that's whats causing the motorical disturbance. And this drug is actually legal.
I think that if you suffer from a depression with hypofrontality, adhd or even negative symptoms of schizophrenia (polypharmacology) Agomelatine might actually work. It shares the same HT-2c antagonism as fluoxetine and some people on fluoxetine tends to get agitated and overactivated from it as well.
I think that hypofrontality also might lead to increased anxiety therefore those people benefitting from them might be benefitting exactly because it's addressing the hypofrontality. Those types of depression I believe is related to those agitated ones. High Anxiety levels, insomnia, lack of appetite, getting angry at everything and eventually ruminating.
Actually always when Ive restarted Fluoxetine I've noticed a difference already after the first dosage. I get pissed from what seems to be nothing, (maybe a memory) triggered from some stimuli, and I usually hit something despite knowing well it wont solve anything. With Fluoxetine I can control that impulse and detect it as a waste of effort.
SSRI however as I mentioned in the letter above is associtated with increased Prolactine , which means less testosterone and Dopamine,, that's why in my case it also causes Iatrogenic anhedoni or accentuates the already existing one.
Your doctor should definitely check your livervalues in the beginning.
If you feel like digging into psychopharmacology I believe the internet is a good source. Wikipedia is often correct but not always and there's a book from Stephen M Stahl Essential Psychopharcology. The new revision seems to be sold in about a year so I would wait for it.
I hope you have success with the drug.
Best regards
Martin
I don't get your point re positive response rate being no better than Prozac, for instance. If each drug helps 33% of ppl, and it's not the same 33%, then that's all to the good, isn't it? More options and higher chance of finding one that works for you. Ten different drugs each helping only 33% of ppl, but not always the same ppl, is like a lottery where each ticket has a one in three chance of winning a prize. If you have ten such tickets, you're a lot more likely to win than if you only have one ticket. The more 33% effective drugs to choose from, the more likely you'll eventually find one where you are among the 33% it helps.
Post a Comment